Our Science
Our science builds on the pioneering work of Nobel laureate Professor Sir Martin Evans in embryonic stem cell research. He was the first to successfully isolate and culture embryonic stem cells from mice, laying the foundational science for genetic engineering in mammals. This work led to the award of the 2007 Nobel Prize for Medicine along with gene editing pioneers Mario Capecchi and Oliver Smithies.
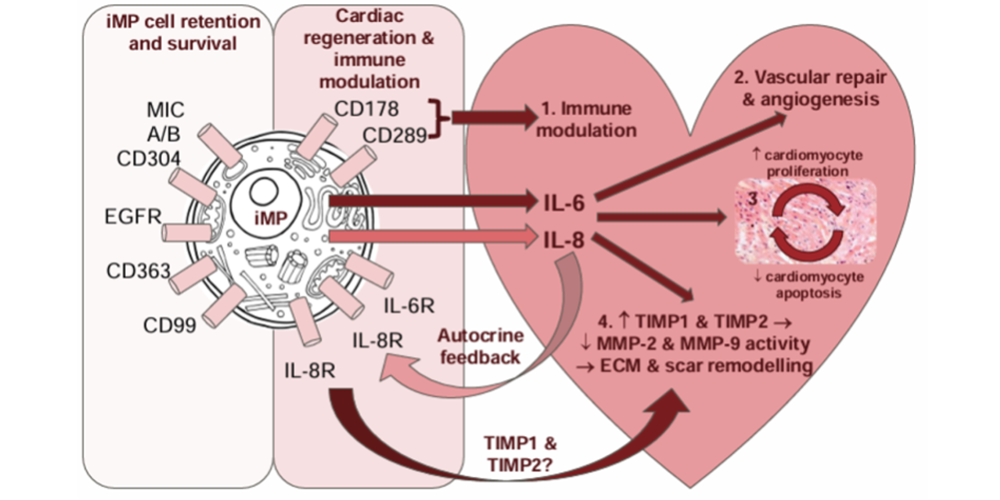
His work in stem cell biology and regenerative medicine paved the way for the innovation that underpinned the development of Cardiogeni’s portfolio of cellular therapies that target the regeneration of damaged heart tissue via a proprietary new cell type known as an immunomodulatory progenitor cell (iMP).
His work informs all aspects of Cardiogeni’s research including:
• Cell culture techniques used to isolate and expand novel cell types, such as the iMP cells.
• Genetic modification methods that inform how iMP cells are engineered for therapeutic uses.
• Regenerative medicine strategies that combine immune modulation with tissue repair.
Our Products
Cardiogeni is developing a breakthrough portfolio of cellular therapies designed to repair damaged heart tissue and restore cardiac function. Our lead candidate, CLXR-001, represents the next generation of regenerative medicine, targeted, potent, and built to transform outcomes for patients with heart failure.
CLXR-001
CLXR-001 addresses the mid-stage heart failure market where patients being treated by coronary artery bypass graft (CABG).
The market consists of approximately 850,000 patients treated annually.
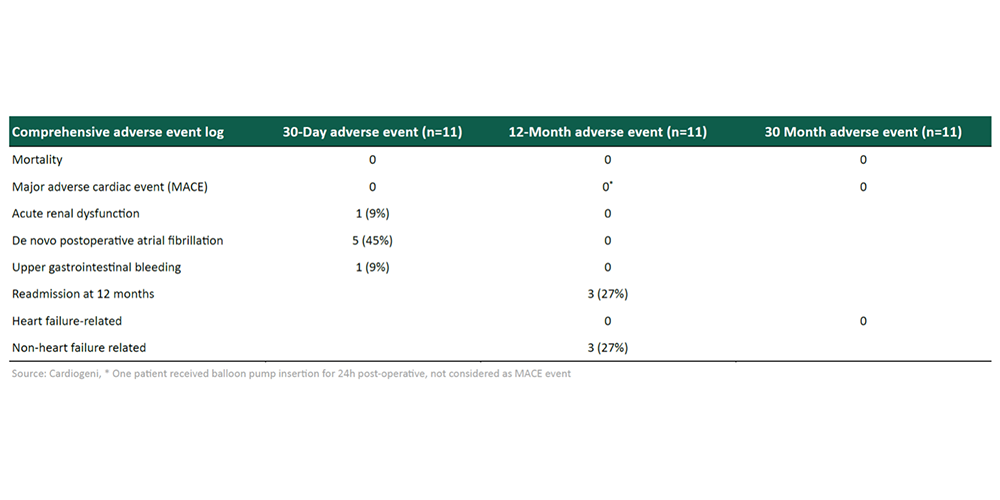
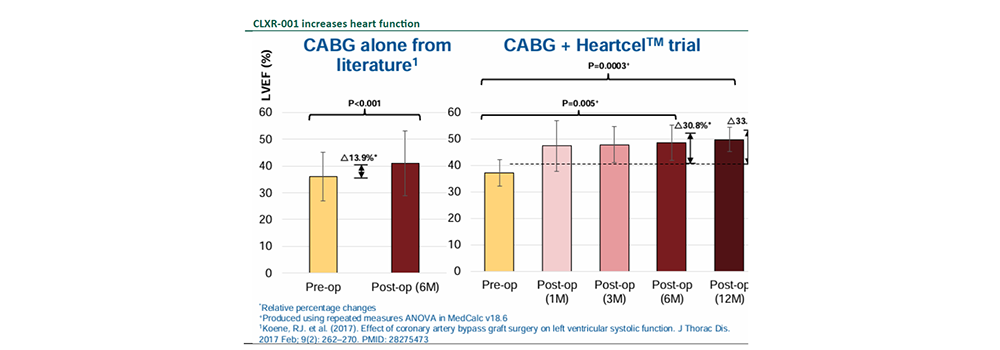
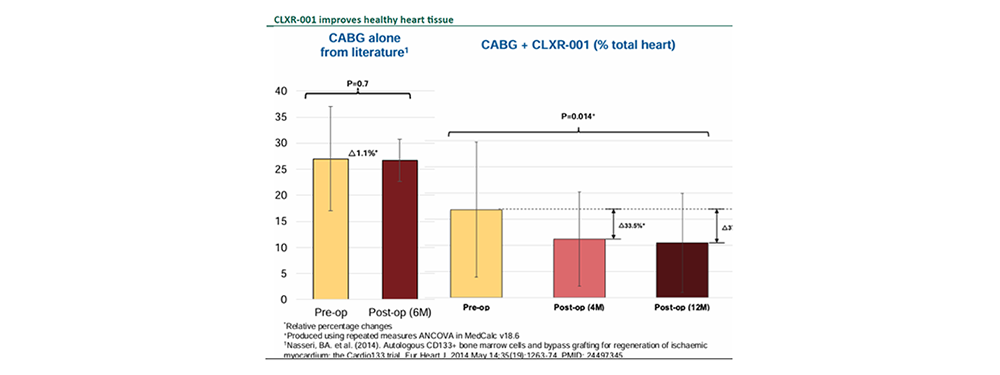
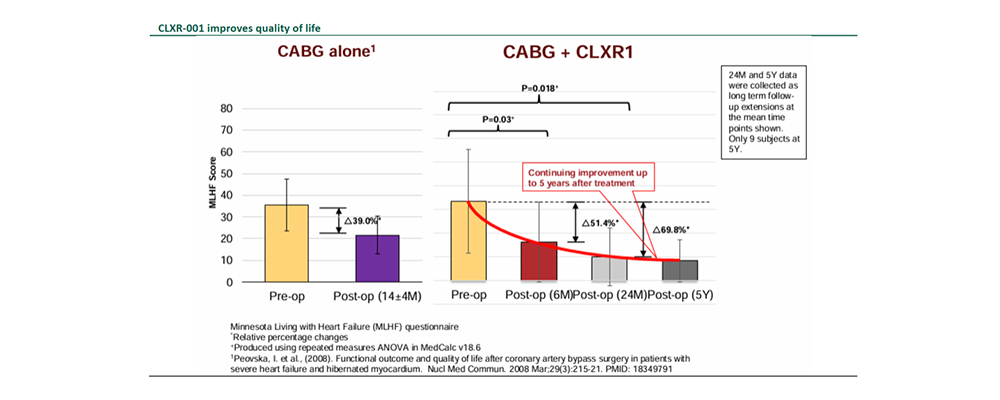
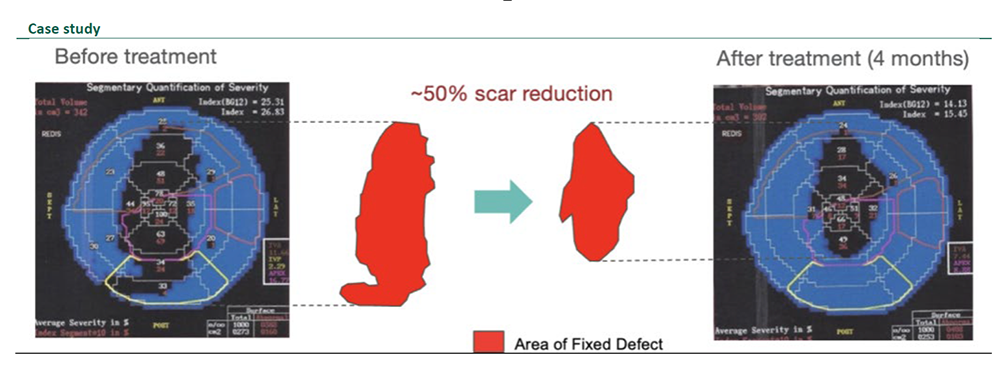
Compelling efficacy and safety data in completed P2a clinical trial.
CLXR-002 & CLXR-003
CLXR-002 and CLXR-003 address the early-stage heart failure market with minimally invasive delivery.
This market consists of approximately 2-3 million patients treated annually.
Compelling pre-clinical animal safety and efficacy data (patch).
CLXR-004
CLXR-004 has been designed for use in combination with a left ventricular assist device (LVAD) device to treat severe heart failure.
The market consists of approximately 3,000 patients treated annually.
LVAD device partners are currently being saught to perform a P1 clinical trial for an orphan indication.
Our Intellectual Property
Cardiogeni’s proprietary technology platform is protected by trade secrets and patents which are all owned by the Company.
Cardiogeni has filed approximately 100 patents and trademarks, with over 30 granted patents in the US, EU and Asia.
Cardiogeni currently has established four patent families:
- Immune-modulatory progenitor (iMP) cells, CLXR-001.
• Progenitor cells of mesodermal origin (PML).
• Mesodermal killer cells (MK) cells.
• Manufacturing Reagents.
These patents safeguard the cell bank, the process and reagents used for modifying cells to produce specific target molecules, and the target molecules themselves. All the cells that are being progressed into clinical testing are protected by Composition of Matter patents